The Ultimate Guide to Terpenes
Terpenes 101: What Are Terpenes? - Introduction
Live Resin Cannabis Derived Terpenes In Vapes & Flower
Live Resin Cannabis Derived Terpenes LRCDT/CDT are used in Utoya products now. While botanical terpenes hold no difference in their own distinct forms, their balance does. What may have the same general terpene profile as something like Pineapple Express (Live Resin Pineapple Express Delta 8 Vape), did not actually come from the cannabis plant itself unless specified.
Utoya’s live resin cannabis-derived terpenes deliver a superior effect for these reasons:
- Actual fresh buds are used
- Solvent-free extraction
- True to the plant driven effects
- Less nosey sneeze-like feelings
- Superior taste
- Exquisite smell
Products that feature live resin terpenes:
- Entourage Vape (THCP Vape)
- Entourage Flower
- Delta 8 Vape
- THCA Vape
- HHC Vape
- The Official Guide To Understanding Terpenes In Hemp And Cannabis Products
Terpenes have been around forever, and are found everywhere. In this article, we will demystify the terpenes, their uses, benefits, and why their presence is so important in the hemp and cannabis industry.
The first and most important thing to understand is that terpenes are considered GRAS, or Generally Regarded As Safe. This means that terpenes do not have any control laws, they are not deemed dangerous or seen as a serious threat to the human body in general use studies approved by the FDA in their ruling. That is not to say that you cannot harm yourself with terpenes or that there is no saturation point of toxicity. We will explore this further in the article.
The second thing, and perhaps equally as important to understand is that terpenes are everywhere. Flowers, plants, and even animals all have and create their own terpenes. Not all of them are useful to us in our hemp and cannabis products. This means that while there may be a specific terpene out there that you would like to get familiar with, it may be incredibly difficult to find or outright impossible depending on your research. Terpenes are only just now getting their attention in our industry by the public audience. Those who have been in the industry for a long time have become more intimately familiar with terpenes and how they work, while others have largely ignored this portion and are just now stepping into the light.
Before we continue talking about all of the wonderful and interesting things that terpenes can do, we need to include this disclaimer. Please read it.
FDA Disclaimer
The contents of this article are for educational purposes only. The FDA has not reviewed any of these statements. This article as well as the contents therein are not meant to diagnose, treat, cure, prevent, or otherwise alleviate any definable medical conditions to any degree of significance. This article is not meant for medical advice. Please speak with your licensed medical professional to get more information regarding terpenes and how they can affect you.
What Is A Terpene?
The term “terpene” was coined in 1866 by the German chemist August Kekulé.
Although sometimes used interchangeably with “terpenes”, terpenoids (or isoprenoids) are modified terpenes that contain additional functional groups, usually oxygen-containing. The name “terpene” is a shortened form of “terpentine”, an obsolete spelling of “turpentine”.
– Source: Wikipedia – Terpene – Terminology
Terpenes (/ˈtɜːrpiːn/) are a class of natural products consisting of compounds with the formula (C5H8)n. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), etc. A well-known monoterpene is alpha-pinene, a major component of turpentine.
Still more numerous than terpenes is a class of compounds called “terpenoids”. Terpenoids are terpenes that have been modified with (usually oxygen-containing) functional groups. The terms terpenes and terpenoids are used interchangeably. Both have strong and often pleasant odors, which may protect their hosts or attract pollinators. The inventory of terpenes and terpenoids is estimated at 55,000 chemical entities.
– Source: Wikipedia – Terpene – Top
How Terpenes Work In Insects, Animals, Plants, and Trees!
Terpenes are major biosynthetic building blocks. Steroids, for example, are derivatives of the triterpene squalene.
Terpenes and terpenoids are also the primary constituents of the essential oils of many types of plants and flowers. In plants, terpenes and terpenoids are important mediators of ecological interactions. For example, they play a role in plant defense against herbivory, disease resistance, the attraction of mutualists such as pollinators, as well as potentially plant-plant communication. They appear to play roles as antifeedants and wound repair.
Higher amounts of terpenes are released by trees in warmer weather, where they may function as a natural mechanism of cloud seeding. The clouds reflect sunlight, allowing the forest temperature to regulate.
Terpenes are also used by insects as a form of defense. For example, termites of the subfamily Nasutitermitinae ward off predatory insects, through the use of a specialized mechanism called a fontanellar gun, which ejects a resinous mixture of terpenes.
Colors and Traits
Terpenes are colorless, although impure samples are often yellow. Boiling points scale with molecular size: terpenes, sesquiterpenes, and diterpenes respectively at 110, 160, and 220 °C. Being highly non-polar, they are insoluble in water. Being hydrocarbons, they are highly flammable and have a low specific gravity (meaning they will float on water).
Terpenoids (mono-, sesqui-, di-, etc.) have similar physical properties but tend to be more polar and hence slightly more soluble in water and somewhat less volatile than their terpene analogs. Highly polar derivatives of terpenoids are glycosides, which are linked to sugars. They are water-soluble solids. They are tactilely light oils considerably less viscous than familiar vegetable oils like corn oil (28 cP), with a viscosity ranging from 1 cP (ala water) to 6 cP. Like other hydrocarbons, they are highly flammable. Terpenes are local irritants and can cause gastrointestinal disturbances if ingested.
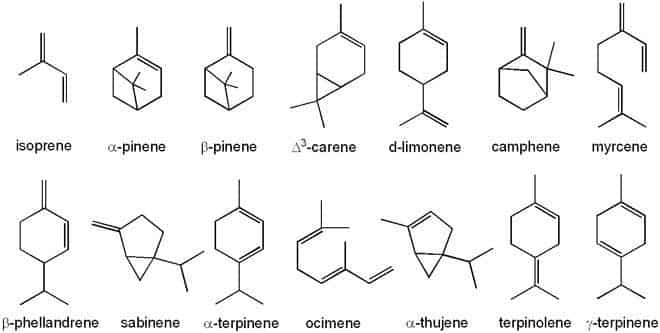
Classifications
Terpenes may be classified by the number of isoprene units in the molecule; a prefix in the name indicates the number of isoprene pairs needed to assemble the molecule. Commonly, terpenes contain 2, 3, 4, or 6 isoprene units; the tetraterpenes (8 isoprene units) form a separate class of compounds called carotenoids; the others are rare. The classification is formalistic only; nothing may be inferred about their properties, uses, or occurrence.
- Hemiterpenes consist of a single isoprene unit. Isoprene itself is considered the only hemiterpene, but oxygen-containing derivatives such as prenol and isovaleric acid are hemiterpenoids.
- Monoterpenes consist of two isoprene units and have the molecular formula C10H16. Examples of monoterpenes and monoterpenoids include geraniol, terpineol (present in lilacs), limonene (present in citrus fruits), myrcene (present in hops), linalool (present in lavender), hinokitiol (present in cypress trees) or pinene (present in pine trees).[20][21] Iridoids derive from monoterpenes. Examples of iridoids include aucubin and catalpol.
- Sesquiterpenes consist of three isoprene units and have the molecular formula C15H24. Examples of sesquiterpenes and sesquiterpenoids include humulene, farnesenes, farnesol, geosmin.[21] (The sesqui- prefix means one and a half.)
- Diterpenes are composed of four isoprene units and have the molecular formula C20H32. They derive from geranylgeranyl pyrophosphate. Examples of diterpenes and diterpenoids are cafestol, kahweol, cembrene, and taxadiene (precursor of taxol). Diterpenes also form the basis for biologically important compounds such as retinol, retinal, and phytol.
- Sesterterpenes, terpenes having 25 carbons and five isoprene units, are rare relative to the other sizes. (The sester- prefix means two and a half.) An example of a sesterterpenoid is geranylfarnesol.
- Triterpenes consist of six isoprene units and have the molecular formula C30H48. The linear triterpene squalene, the major constituent of shark liver oil, is derived from the reductive coupling of two molecules of farnesyl pyrophosphate. Squalene is then processed biosynthetically to generate either lanosterol or cycloartenol, the structural precursors to all the steroids.
- Sesquiterpenes are composed of seven isoprene units and have the molecular formula C35H56. Sesquiterpenes are typically microbial in their origin. Examples of sesquiterpenoids are ferrugicadiol and tetraprenylcurcumene.
- Tetraterpenes contain eight isoprene units and have the molecular formula C40H64. Biologically important tetraterpenoids include the acyclic lycopene, the monocyclic gamma-carotene, and the bicyclic alpha- and beta-carotenes.
- Polyterpenes consist of long chains of many isoprene units. Natural rubber consists of polyisoprene in which the double bonds are cis. Some plants produce a polyisoprene with trans double bonds, known as gutta-percha.
- Norisoprenoids, such as the C13-norisoprenoid 3-oxo-α-ionol present in Muscat of Alexandria leaves and 7,8-dihydroionone derivatives, such as megastigmane-3,9-diol and 3-oxo-7,8-dihydro-α-ionol found in Shiraz leaves (both grapes in the species Vitis vinifera) or wine (responsible for some of the spice notes in Chardonnay), can be produced by fungal peroxidases[25] or glycosidases.[26]
Read the study – Terpenes and the Coronavirus
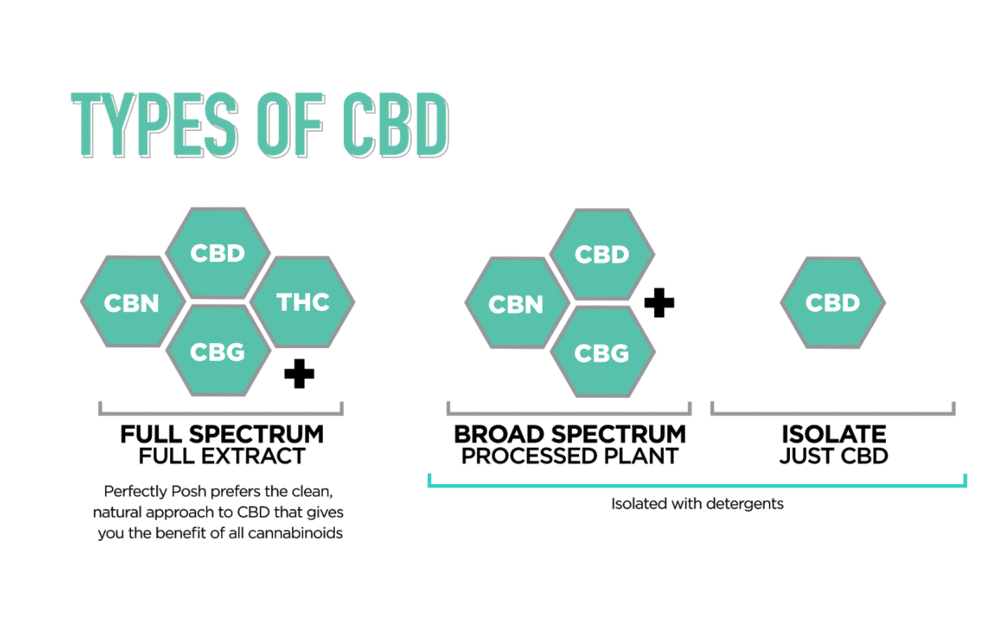
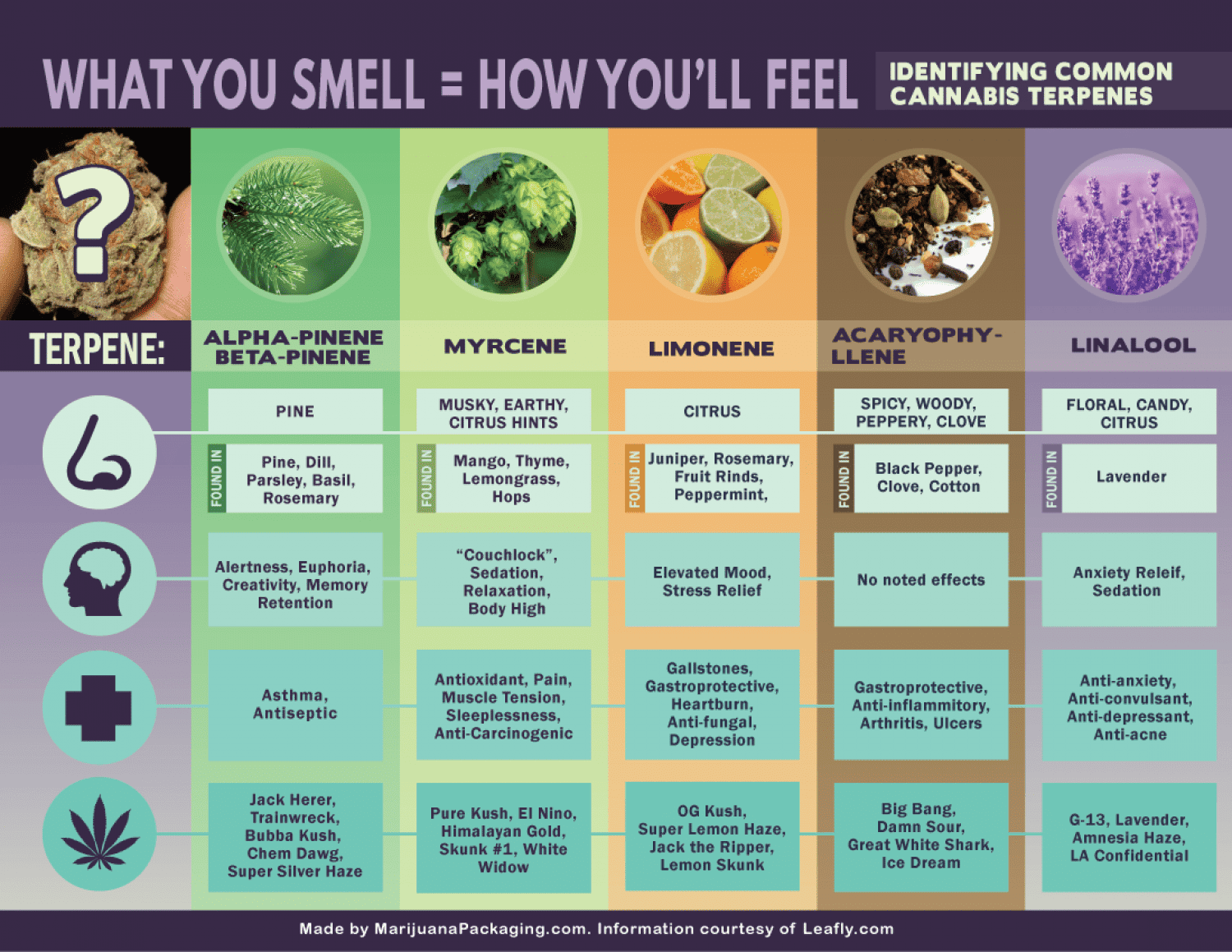
Hemp and Cannabis Chart of Terpenes
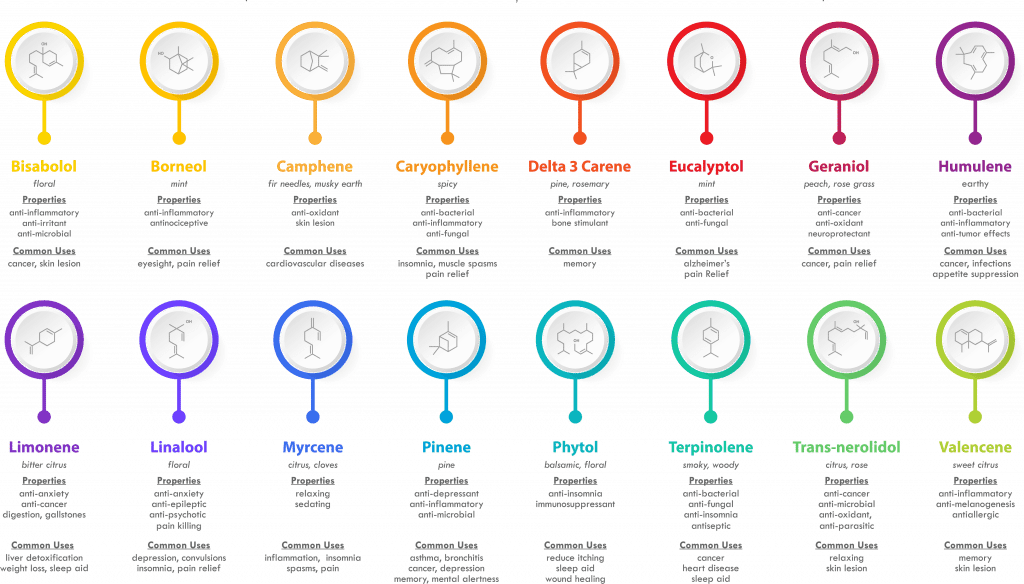
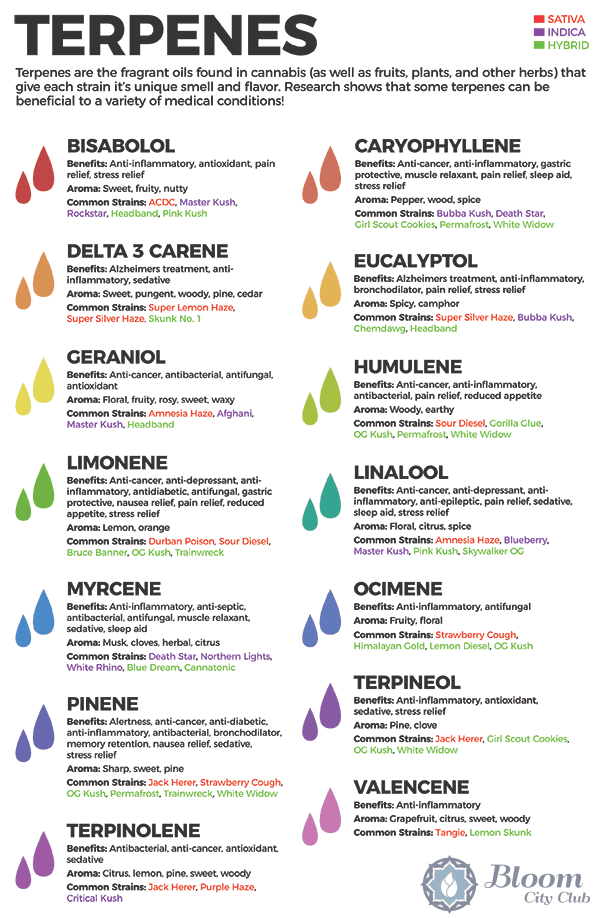
10 Hemp/Cannabis Terpenes And What They Do
LIMONENE
This terpene is most commonly found in citrus fruits like lemons, limes, and oranges. It’s been tied to everything from reduced stress to increased energy; it may also be a natural digestive aid. Like many other terpenes, limonene seems to trigger enzymes that turn on fat burning when it’s ingested. And fat burning is the stablest form of energy production there is.
Many people credit limonene-heavy essential oils by giving them an improved clarity of mind. Hemp strains high in limonene have a similar effect.
PINENE
The namesake terpene in pine trees, pinene is a known bronchodilator. That just means it opens up the lungs and makes you better at breathing! This quality makes pinene especially helpful to people with asthma.
Pinene is also calming. You can experience these same benefits by smoking or vaping hemp strains like Trophy Wife or Jack Herer. Bonus points if you decide to partake while walking through a pine forest.
LINALOOL
Lavender, the calming and soothing smell is a terpene. Linalool is both relaxing and anti-inflammatory, and it actually calms down the nervous system to promote better health. Some research shows that linalool activates the same TRPA and TRPV channels CBD does, resulting in less inflammation and pain.
GUAIOL
Guaiol isn’t as well-known as some terpenes, but maybe it should be. This terp shares a lot in common with pinene. It gets its name from the guaiacum plant whose bark it’s derived from; like pinene, guaiol smells woodsy and earthy.
Research shows that guaiol may be both anti-viral and anti-bacterial.
EUCALYPTOL
Eucalyptol is a terpene often found in eucalyptus, salvia, and hemp. Studies show it may help regulate the immune system and activate one’s innate immune response. Translation: eucalyptol could help one’s immune system avoid over-responding to short-term issues by strengthening things from the ground up.
BETA-CARYOPHYLLENE
This terpene is an interesting one. While beta-caryophyllene is commonly found within black pepper and cloves, it also makes an appearance in hemp. And research shows it binds to the very same endocannabinoid receptors as CBD — so much that researchers consider it a “dietary cannabinoid.”
This terpene is associated with reduced inflammation and a healthier nervous system.
BISABOLOL
Bisabolol is usually found in chamomile flowers. Research shows it’s anti-inflammatory, anti-bacterial, and more. Bisabolol is said to have a delicate floral scent.
MYRCENE
Myrcene’s presence is felt pretty much everywhere. It’s found in mangoes, thyme, basil, and hops. And hops’ botanical cousin hemp, of course. Many hemp and cannabis users favor this terpene since it plays a role in what is commonly called “couch lock.” Hemp’s myrcene content also provides relaxing results. Myrcene may reduce inflammation, boost immunity, and calm the nervous system enough to help you get the sleep you need.
CAMPHENE
Camphene might be a “minor” terpene, but what it lacks in popularity, it makes up for in benefits. Camphene may do everything from reducing cholesterol levels to protect against infection. Think of camphene’s scent as a combination of pinene’s and myrcene’s. It’s an unusual smell. This terpene is not easy to come by, and perhaps it is for good reason, you can always have too much of even a good thing.
TERPINOLENE
Finally, we end our list at terpinolene. This terpene has a complex sweet + spicy scent and can be found in apples, lilacs, nutmeg, allspice, and more. Some of the more ‘earthy’ strains of hemp feature high levels of pinene and terpinolene in combination. If ingested, it may slow the growth of harmful cells, at least according to this 2013 study. True to its scent, terpinolene may also help by calming the nervous system.
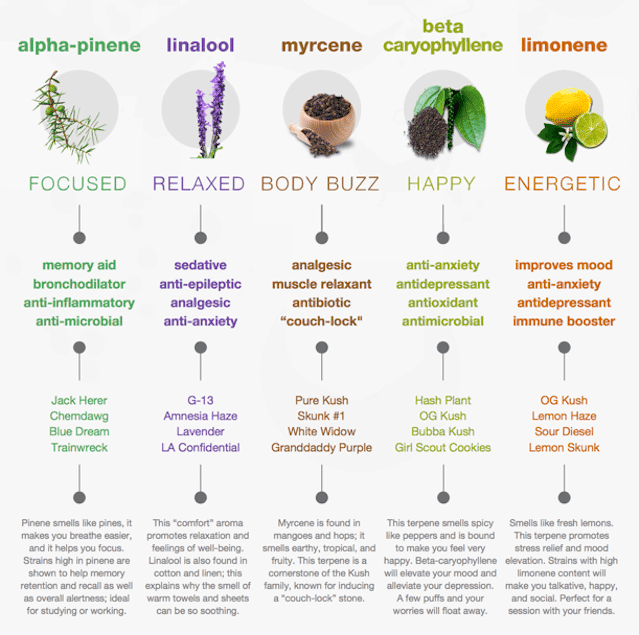
Common Terpenes To Remember & Some of their Cannabis Associates
Shopping With Your New Knowledge In Mind:
When looking to make your purchases, now that you are more educated in how the terpene profile can affect how you feel, it’s time to make more educated decisions on what you would like to feel like for future sessions, while browsing through our selection of hot hemp delta 8 flowers.
You can take a very basic approach to terpene profiles by understanding the most core basics of their effects. The best way to figure out what you want, is going to start by asking yourself some questions:
- You know what wakes you up. Which strains will wake me up? (Super Sour Space Candy)
- You know what relaxes you. Which strains will relax you? (Suver Haze)
- You know which strains will balance you. Which strain has a little of everything? (Lifter)
- You know which strains have more complex profiles. Which strains have more sides to them due to their cannabinoid profiles? (White CBG)
Myths and Lies About To Avoid Repeating Or Believing:
- Terpenes are not harmful. This is not true. Terpenes, depending on their chemical structure and source, can be harmful to plants, animals, or people. Only so many terpenes are listed as GRAS.
- Terpenes are only made from Cannabis. This is not true. Cannabis contains and creates terpenes, but it is not the only thing that creates terpenes. Many things have terpenes, including plants, fruits, animals, and insects.
- Terpenes are the same thing as turpentine. This is not true. Turpentine is quite different and is poisonous to humans. Turpentine is extracted from the pine tree.
- Terpenes are medicinal. This is not accurate. Many terpenes are considered GRAS, therefore, by law, they cannot be considered medicinal or labeled as such.
- You cannot overdose on terpenes / there is no terpene toxicity. This is not 100% true. While terpenes that are naturally present in cannabinoids to their Too much of one terpene can cause dizziness, nausea, and confusion. If you feel you have been exposed to an abundance of terpenes, you may call poison control for guidance at 888-222-1222. You can also use their online database to research anything you might be ingesting or experiencing. Terpenes exhibit auto-toxicity, meaning they are toxic even to the plant that produces them. That is why plants like cannabis store them in trichomes, or in other special glands that are meant to hold them safely. While terpenes are highly microbicidal, cannabis-derived terpenes considered safe for human use and consumption within the concentrations in which they are normally present, particularly in their topical use as modifiers of skin tissue permeability.
- Terpenes Extracted From Cannabis Plants Are More Powerful or Useful Than Botanical Terpenes Extracted From Fruits & Other Plants. False. Botanically extracted terpenes, by definition, is no different at all than extracting terpenes from a botanical plant we call cannabis or hemp. Botanical terpenes are just as efficacious as cannabis terpenes. Plant-derived terpenes can catalyze the entourage effect and potentiate cannabinoids to work better in the body after consumption. That is not to say that there are not times when someone might want to use a cannabis-derived terpene over a non-cannabis botanical terpene. These questions typically arise during product formulation, where it must be decided by the manufacturer or creator how the terpenes are going to be applied (ingested or topical), and what the desirable effects may be. Some companies simply want to have the title say cannabis-derived terpenes and mean it. It’s not a bad thing, but don’t believe that terpenes are going to be less valuable because one linalool came from a flower and the other came from a cannabis flower.
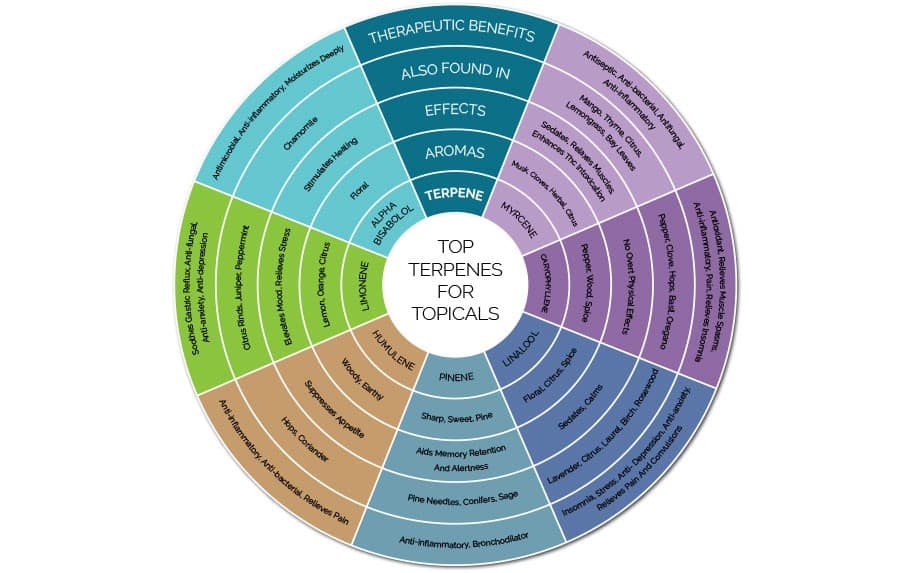
Additional Charts / Infographics:
These are some additional visual learning aids to help you further understand terpenes. While these charts are present and available, it is always best to use your best intuition and read more about what you are using individually. Terpenes are an amazing science, and we hope that this article inspires you to learn more!
- The Official Guide To Understanding Terpenes In Hemp And Cannabis Products
Terpenes have been around forever, and are found everywhere. In this article, we will demystify the terpenes, their uses, benefits, and why their presence is so important in the hemp and cannabis industry.
The first and most important thing to understand is that terpenes are considered GRAS, or Generally Regarded As Safe. This means that terpenes do not have any control laws, they are not deemed dangerous or seen as a serious threat to the human body in general use studies approved by the FDA in their ruling. That is not to say that you cannot harm yourself with terpenes or that there is no saturation point of toxicity. We will explore this further in the article.
The second thing, and perhaps equally as important to understand is that terpenes are everywhere. Flowers, plants, and even animals all have and create their own terpenes. Not all of them are useful to us in our hemp and cannabis products. This means that while there may be a specific terpene out there that you would like to get familiar with, it may be incredibly difficult to find or outright impossible depending on your research. Terpenes are only just now getting their attention in our industry by the public audience. Those who have been in the industry for a long time have become more intimately familiar with terpenes and how they work, while others have largely ignored this portion and are just now stepping into the light.
Before we continue talking about all of the wonderful and interesting things that terpenes can do, we need to include this disclaimer. Please read it.
FDA Disclaimer
The contents of this article are for educational purposes only. The FDA has not reviewed any of these statements. This article as well as the contents therein are not meant to diagnose, treat, cure, prevent, or otherwise alleviate any definable medical conditions to any degree of significance. This article is not meant for medical advice. Please speak with your licensed medical professional to get more information regarding terpenes and how they can affect you.
What Is A Terpene?
The term “terpene” was coined in 1866 by the German chemist August Kekulé.
Although sometimes used interchangeably with “terpenes”, terpenoids (or isoprenoids) are modified terpenes that contain additional functional groups, usually oxygen-containing. The name “terpene” is a shortened form of “terpentine”, an obsolete spelling of “turpentine”.
– Source: Wikipedia – Terpene – Terminology
Terpenes (/ˈtɜːrpiːn/) are a class of natural products consisting of compounds with the formula (C5H8)n. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), etc. A well-known monoterpene is alpha-pinene, a major component of turpentine.
Still more numerous than terpenes is a class of compounds called “terpenoids”. Terpenoids are terpenes that have been modified with (usually oxygen-containing) functional groups. The terms terpenes and terpenoids are used interchangeably. Both have strong and often pleasant odors, which may protect their hosts or attract pollinators. The inventory of terpenes and terpenoids is estimated at 55,000 chemical entities.
– Source: Wikipedia – Terpene – Top
How Terpenes Work In Insects, Animals, Plants, and Trees!
Terpenes are major biosynthetic building blocks. Steroids, for example, are derivatives of the triterpene squalene.
Terpenes and terpenoids are also the primary constituents of the essential oils of many types of plants and flowers. In plants, terpenes and terpenoids are important mediators of ecological interactions. For example, they play a role in plant defense against herbivory, disease resistance, the attraction of mutualists such as pollinators, as well as potentially plant-plant communication. They appear to play roles as antifeedants and wound repair.
Higher amounts of terpenes are released by trees in warmer weather, where they may function as a natural mechanism of cloud seeding. The clouds reflect sunlight, allowing the forest temperature to regulate.
Terpenes are also used by insects as a form of defense. For example, termites of the subfamily Nasutitermitinae ward off predatory insects, through the use of a specialized mechanism called a fontanellar gun, which ejects a resinous mixture of terpenes.
Colors and Traits
Terpenes are colorless, although impure samples are often yellow. Boiling points scale with molecular size: terpenes, sesquiterpenes, and diterpenes respectively at 110, 160, and 220 °C. Being highly non-polar, they are insoluble in water. Being hydrocarbons, they are highly flammable and have a low specific gravity (meaning they will float on water).
Terpenoids (mono-, sesqui-, di-, etc.) have similar physical properties but tend to be more polar and hence slightly more soluble in water and somewhat less volatile than their terpene analogs. Highly polar derivatives of terpenoids are glycosides, which are linked to sugars. They are water-soluble solids. They are tactilely light oils considerably less viscous than familiar vegetable oils like corn oil (28 cP), with a viscosity ranging from 1 cP (ala water) to 6 cP. Like other hydrocarbons, they are highly flammable. Terpenes are local irritants and can cause gastrointestinal disturbances if ingested.
Classifications
Terpenes may be classified by the number of isoprene units in the molecule; a prefix in the name indicates the number of isoprene pairs needed to assemble the molecule. Commonly, terpenes contain 2, 3, 4, or 6 isoprene units; the tetraterpenes (8 isoprene units) form a separate class of compounds called carotenoids; the others are rare. The classification is formalistic only; nothing may be inferred about their properties, uses, or occurrence.
- Hemiterpenes consist of a single isoprene unit. Isoprene itself is considered the only hemiterpene, but oxygen-containing derivatives such as prenol and isovaleric acid are hemiterpenoids.
- Monoterpenes consist of two isoprene units and have the molecular formula C10H16. Examples of monoterpenes and monoterpenoids include geraniol, terpineol (present in lilacs), limonene (present in citrus fruits), myrcene (present in hops), linalool (present in lavender), hinokitiol (present in cypress trees) or pinene (present in pine trees).[20][21] Iridoids derive from monoterpenes. Examples of iridoids include aucubin and catalpol.
- Sesquiterpenes consist of three isoprene units and have the molecular formula C15H24. Examples of sesquiterpenes and sesquiterpenoids include humulene, farnesenes, farnesol, geosmin.[21] (The sesqui- prefix means one and a half.)
- Diterpenes are composed of four isoprene units and have the molecular formula C20H32. They derive from geranylgeranyl pyrophosphate. Examples of diterpenes and diterpenoids are cafestol, kahweol, cembrene, and taxadiene (precursor of taxol). Diterpenes also form the basis for biologically important compounds such as retinol, retinal, and phytol.
- Sesterterpenes, terpenes having 25 carbons and five isoprene units, are rare relative to the other sizes. (The sester- prefix means two and a half.) An example of a sesterterpenoid is geranylfarnesol.
- Triterpenes consist of six isoprene units and have the molecular formula C30H48. The linear triterpene squalene, the major constituent of shark liver oil, is derived from the reductive coupling of two molecules of farnesyl pyrophosphate. Squalene is then processed biosynthetically to generate either lanosterol or cycloartenol, the structural precursors to all the steroids.
- Sesquiterpenes are composed of seven isoprene units and have the molecular formula C35H56. Sesquiterpenes are typically microbial in their origin. Examples of sesquiterpenoids are ferrugicadiol and tetraprenylcurcumene.
- Tetraterpenes contain eight isoprene units and have the molecular formula C40H64. Biologically important tetraterpenoids include the acyclic lycopene, the monocyclic gamma-carotene, and the bicyclic alpha- and beta-carotenes.
- Polyterpenes consist of long chains of many isoprene units. Natural rubber consists of polyisoprene in which the double bonds are cis. Some plants produce a polyisoprene with trans double bonds, known as gutta-percha.
- Norisoprenoids, such as the C13-norisoprenoid 3-oxo-α-ionol present in Muscat of Alexandria leaves and 7,8-dihydroionone derivatives, such as megastigmane-3,9-diol and 3-oxo-7,8-dihydro-α-ionol found in Shiraz leaves (both grapes in the species Vitis vinifera) or wine (responsible for some of the spice notes in Chardonnay), can be produced by fungal peroxidases[25] or glycosidases.[26]

Read the study – Terpenes and the Coronavirus
Hemp and Cannabis Chart of Terpenes

10 Hemp/Cannabis Terpenes And What They Do
LIMONENE
This terpene is most commonly found in citrus fruits like lemons, limes, and oranges. It’s been tied to everything from reduced stress to increased energy; it may also be a natural digestive aid. Like many other terpenes, limonene seems to trigger enzymes that turn on fat burning when it’s ingested. And fat burning is the stablest form of energy production there is.
Many people credit limonene-heavy essential oils by giving them an improved clarity of mind. Hemp strains high in limonene have a similar effect.
PINENE
The namesake terpene in pine trees, pinene is a known bronchodilator. That just means it opens up the lungs and makes you better at breathing! This quality makes pinene especially helpful to people with asthma.
Pinene is also calming. You can experience these same benefits by smoking or vaping hemp strains like Trophy Wife or Jack Herer. Bonus points if you decide to partake while walking through a pine forest.
LINALOOL
Lavender, the calming and soothing smell is a terpene. Linalool is both relaxing and anti-inflammatory, and it actually calms down the nervous system to promote better health. Some research shows that linalool activates the same TRPA and TRPV channels CBD does, resulting in less inflammation and pain.
GUAIOL
Guaiol isn’t as well-known as some terpenes, but maybe it should be. This terp shares a lot in common with pinene. It gets its name from the guaiacum plant whose bark it’s derived from; like pinene, guaiol smells woodsy and earthy.
Research shows that guaiol may be both anti-viral and anti-bacterial.
EUCALYPTOL
Eucalyptol is a terpene often found in eucalyptus, salvia, and hemp. Studies show it may help regulate the immune system and activate one’s innate immune response. Translation: eucalyptol could help one’s immune system avoid over-responding to short-term issues by strengthening things from the ground up.
BETA-CARYOPHYLLENE
This terpene is an interesting one. While beta-caryophyllene is commonly found within black pepper and cloves, it also makes an appearance in hemp. And research shows it binds to the very same endocannabinoid receptors as CBD — so much that researchers consider it a “dietary cannabinoid.”
This terpene is associated with reduced inflammation and a healthier nervous system.
BISABOLOL
Bisabolol is usually found in chamomile flowers. Research shows it’s anti-inflammatory, anti-bacterial, and more. Bisabolol is said to have a delicate floral scent.
MYRCENE
Myrcene’s presence is felt pretty much everywhere. It’s found in mangoes, thyme, basil, and hops. And hops’ botanical cousin hemp, of course. Many hemp and cannabis users favor this terpene since it plays a role in what is commonly called “couch lock.” Hemp’s myrcene content also provides relaxing results. Myrcene may reduce inflammation, boost immunity, and calm the nervous system enough to help you get the sleep you need.
CAMPHENE
Camphene might be a “minor” terpene, but what it lacks in popularity, it makes up for in benefits. Camphene may do everything from reducing cholesterol levels to protect against infection. Think of camphene’s scent as a combination of pinene’s and myrcene’s. It’s an unusual smell. This terpene is not easy to come by, and perhaps it is for good reason, you can always have too much of even a good thing.
TERPINOLENE
Finally, we end our list at terpinolene. This terpene has a complex sweet + spicy scent and can be found in apples, lilacs, nutmeg, allspice, and more. Some of the more ‘earthy’ strains of hemp feature high levels of pinene and terpinolene in combination. If ingested, it may slow the growth of harmful cells, at least according to this 2013 study. True to its scent, terpinolene may also help by calming the nervous system.
Common Terpenes To Remember & Some of their Cannabis Associates

Shopping With Your New Knowledge In Mind:
When looking to make your purchases, now that you are more educated in how the terpene profile can affect how you feel, it’s time to make more educated decisions on what you would like to feel like for future sessions, while browsing through our selection of hot hemp delta 8 flowers.
You can take a very basic approach to terpene profiles by understanding the most core basics of their effects. The best way to figure out what you want, is going to start by asking yourself some questions:
- You know what wakes you up. Which strains will wake me up? (Super Sour Space Candy)
- You know what relaxes you. Which strains will relax you? (Suver Haze)
- You know which strains will balance you. Which strain has a little of everything? (Lifter)
- You know which strains have more complex profiles. Which strains have more sides to them due to their cannabinoid profiles? (White CBG)
Myths and Lies About To Avoid Repeating Or Believing:
- Terpenes are not harmful. This is not true. Terpenes, depending on their chemical structure and source, can be harmful to plants, animals, or people. Only so many terpenes are listed as GRAS.
- Terpenes are only made from Cannabis. This is not true. Cannabis contains and creates terpenes, but it is not the only thing that creates terpenes. Many things have terpenes, including plants, fruits, animals, and insects.
- Terpenes are the same thing as turpentine. This is not true. Turpentine is quite different and is poisonous to humans. Turpentine is extracted from the pine tree.
- Terpenes are medicinal. This is not accurate. Many terpenes are considered GRAS, therefore, by law, they cannot be considered medicinal or labeled as such.
- You cannot overdose on terpenes / there is no terpene toxicity. This is not 100% true. While terpenes that are naturally present in cannabinoids to their Too much of one terpene can cause dizziness, nausea, and confusion. If you feel you have been exposed to an abundance of terpenes, you may call poison control for guidance at 888-222-1222. You can also use their online database to research anything you might be ingesting or experiencing. Terpenes exhibit auto-toxicity, meaning they are toxic even to the plant that produces them. That is why plants like cannabis store them in trichomes, or in other special glands that are meant to hold them safely. While terpenes are highly microbicidal, cannabis-derived terpenes considered safe for human use and consumption within the concentrations in which they are normally present, particularly in their topical use as modifiers of skin tissue permeability.
- Terpenes Extracted From Cannabis Plants Are More Powerful or Useful Than Botanical Terpenes Extracted From Fruits & Other Plants. False. Botanically extracted terpenes, by definition, is no different at all than extracting terpenes from a botanical plant we call cannabis or hemp. Botanical terpenes are just as efficacious as cannabis terpenes. Plant-derived terpenes can catalyze the entourage effect and potentiate cannabinoids to work better in the body after consumption. That is not to say that there are not times when someone might want to use a cannabis-derived terpene over a non-cannabis botanical terpene. These questions typically arise during product formulation, where it must be decided by the manufacturer or creator how the terpenes are going to be applied (ingested or topical), and what the desirable effects may be. Some companies simply want to have the title say cannabis-derived terpenes and mean it. It’s not a bad thing, but don’t believe that terpenes are going to be less valuable because one linalool came from a flower and the other came from a cannabis flower.
Additional Charts / Infographics:
These are some additional visual learning aids to help you further understand terpenes. While these charts are present and available, it is always best to use your best intuition and read more about what you are using individually. Terpenes are an amazing science, and we hope that this article inspires you to learn more!




Terpenes 101: Part 2 - Terpene Blends In CBD And Delta 8 Oils
Terpenes are commonly used throughout the Hemp Industry, primarily in things like delta 8 vape carts and cbd oils. These two products are incredibly popular, and serve many people today!
It is not the only thing that terpenes have been added too. In fact, there are several other applications that terpenes have in the world of hemp and cannabis, including skincare and fragrances!
The Entourage Effect & Terpenes
The entourage effect is when cannabinoids and terpenes work together to bolster one another’s effects in the body.
Terpenes play a very important role in how cannabis cannabinoids will make us feel.
A popular misconception is that the higher the THC in a strain of medical Cannabis will produce better effects than Cannabis with a lower amount of THC. This is incorrect. The higher the THC content, you will likely have higher psychoactive effects, this much is easy enough to understand. We know that the more THC is present, the more we will feel it in our heads.
So what is the misconception?
Terpenes play an important role in how you will feel more so than the cannabinoid will. Let’s do an example together below.
Let’s pretend for a moment that your favorite strain is an Indica dominant strain. We will pretend that this is Skywalker for now.
Skywalker flower typically has THC percentages ranging from 14% – 23%. It’s high in myrcene
Let’s give it these statistics:
< 0.01% Linalool
0.42% Caryophyllene oxide
0.69% Myrcene
< 0.01% beta-Pinene
< 0.01% Limonene
< 0.01% Terpinolene
0.88% alpha-Pinene
1.77% Humulene
0.37% Caryophyllene
4.13% TERPENE-TOTAL
Now let’s take a similar profile but with a little less kick
Let’s take Hash Plant as our comparison.
Hash Plant typically ranges between 13% – 19%, a little less than its Skywalker competitor.
We will give it these statistics:
*Beta-Myrcene: 1.317%
*Trans-Carophyllene: 0.377%
Alpha-Pinene: 0.311%
Beta-Pinene: 0.112%
R-Limonene: 0.239%
Humulene: 0.125%
Nerolidol-2: 0.050%
Ocimene-2: 0.190%
Total Terpenes: 2.721%
Now that we have these two comparisons up, of the two, if your goal was to get completely couch-locked and relax, which strain would do this more for you?
You will ultimately feel slightly more awake on the Skywalker and you will get much more sleepy effects out of the Hash Plant. Why? The terpene profile makes it work.
The skywalker may have a higher cannabinoid content, but its terpene profile will not satisfy the sleep goal quite as well as the Hash Plant given the ratio difference in Myrcene and the variety of terpenes included in Hashplant are much more balanced to sedate than the Skywalker. The Trans-Caryophyllene also plays an important role in aiding in sedation given its interactions with the CB1 and CB2 receptors, working to relax the body. Ocimene-2 has some interesting studied effects as well, though primarily anti-inflammatory, it has anti-oxidative properties as well as the ability to inhibit key enzymes connected to type 2 diabetes and hypertension.
Very quickly you can see how just a few minor things involved specifically in terpenes can change your overall experience. It’s always good to pay attention to both.
Something to help you remember a little bit about terpenes and cannabinoids is that they both come from the same thing. Trichomes. Those sparkly little sugary diamonds on top of the buds are what produce these. Look at the flower you are inspecting and observe its trichome coating. This can give you a good visual idea about the type of potency you are dealing with. The more trichomes there are, the more cannabinoids and terpenes are present.
You can apply this same general information to our hot hemp. The strains that we have provided all come with terpene profiles to help you get a better understanding of the kind of experience you will have with each flower!
“Cannabinoids are the powerful engine, and cannabinoids are the steering wheel.”
Imagine a manufacturer of vehicles.
This vehicle manufacturer creates a variety of options to allow the consumer a choice of experience. Each vehicle is a little different than the last. One vehicle might have a v8 engine with a ton of power, nice features, and a beautiful interior. Others may not be so attractive and might not have as many bells and whistles to it but will get you from place to place.
Now imagine that the engine in each vehicle is powered by cannabinoids such as THC, CBD, CBN, etc.
Now let’s imagine that in each and every one of these vehicles there is a steering wheel and a GPS navigation system. Each vehicle has a pre-determined set of instructions that say where to go, how fast, and can even have an effect on how long you are there for. Let’s imagine that these steering wheels and GPS navigators are the terpenes such as Myrcene, Linalool, Beta-Caryophyllene, etc.
Each vehicle will take you to a different destination and each destination will feel differently.
Now let’s transform this imaginary factory into a cannabis farm.
The only difference now is that rather than destinations and time with cars, you have effects and how long they will last depending on the strain or mixture of cannabinoids and terpenes that you have chosen for yourself.